A recent study conducted by researchers at Purdue University has identified fat deposits in brain cells as a significant factor in the progression of Alzheimer’s disease. This discovery suggests that targeting these fat accumulations may present a new potential avenue for treatment, diverging from the traditional focus on protein clumps associated with the disease.
Research surrounding Alzheimer’s has predominantly emphasized the abnormal accumulation of proteins, such as amyloid beta, which are believed to contribute to neurodegeneration. While various attempts to target these clumps have yielded mixed results, some scientists are now looking for additional components contributing to the disease’s progression. According to Gaurav Chopra, a chemist at Purdue University, “In our view, directly targeting plaques or tangles will not solve the problem; we need to restore the function of immune cells in the brain.”
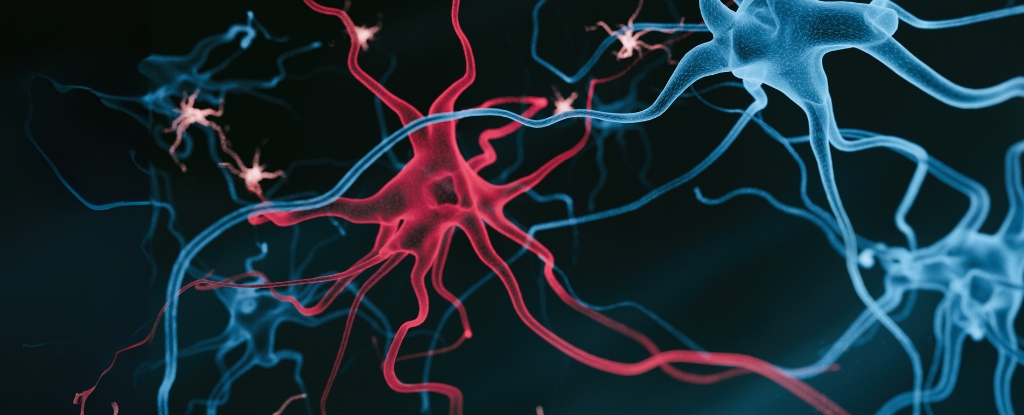
The study highlights how immune cells, specifically astrocytes and microglia, are disrupted by associated fat molecules. Researchers found that amyloid beta protein clumps might not be the primary cause of the disease but rather a symptom of underlying issues.
Impact of Fat Accumulation on Microglial Function
In their investigation, neuroscientist Priya Prakash and colleagues examined brain tissue from Alzheimer’s patients to evaluate the effects of fat accumulation on microglial cells. They discovered that when these supportive immune cells are positioned near amyloid beta plaques—within a distance of 10 micrometers—they accumulate lipid deposits that impair their ability to clear the plaques. The study indicated that this buildup leads to a staggering 40 percent reduction in the plaque-clearing capacity of microglia compared to healthy cells.
Chopra noted, “We showed that amyloid beta is directly responsible for the fat that forms inside microglia. Because of these fatty deposits, microglial cells become dysfunctional—they stop clearing amyloid beta and stop doing their job.”
The researchers pinpointed the enzyme responsible for this excessive fat storage as DGAT2. Targeting this enzyme could offer a new therapeutic strategy for Alzheimer’s treatment. Chopra elaborated, “What we’ve seen is that when we target the fat-making enzyme and either remove or degrade it, we restore the microglia’s ability to fight disease and maintain balance in the brain—which is what they’re meant to do.”
Broader Implications for Alzheimer’s Treatment
The implications of this research are significant, particularly in light of the growing global burden of dementia. Each year, approximately 10 million new cases of dementia are diagnosed worldwide. Despite decades of research, effective treatment options remain elusive, making the exploration of new strategies essential for improving patient outcomes.
Palak Manchanda, a neuroimmunologist at Purdue University, stated, “By pinpointing this lipid burden and the DGAT2 switch that drives it, we reveal a completely new therapeutic angle. Restore microglial metabolism and you may restore the brain’s own defense against disease.”
The findings of this research were published in the journal Immunity, marking a pivotal moment in the quest for effective Alzheimer’s treatments. As the scientific community continues to explore these newly identified mechanisms, there is hope that innovative therapies targeting fat accumulation in the brain may ultimately enhance the quality of life for individuals affected by this debilitating disease.