A groundbreaking study reveals that magnetoelectric nanoparticles (MENPs) can effectively target and reduce pancreatic tumors in preclinical models, significantly extending survival times. Conducted by a team from the **Sylvester Comprehensive Cancer Center**, **University of Miami College of Engineering**, **Moffitt Cancer Center**, and **Cellular Nanomed, Inc.**, the findings were published in the **November 3, 2025**, issue of **Advanced Science**.
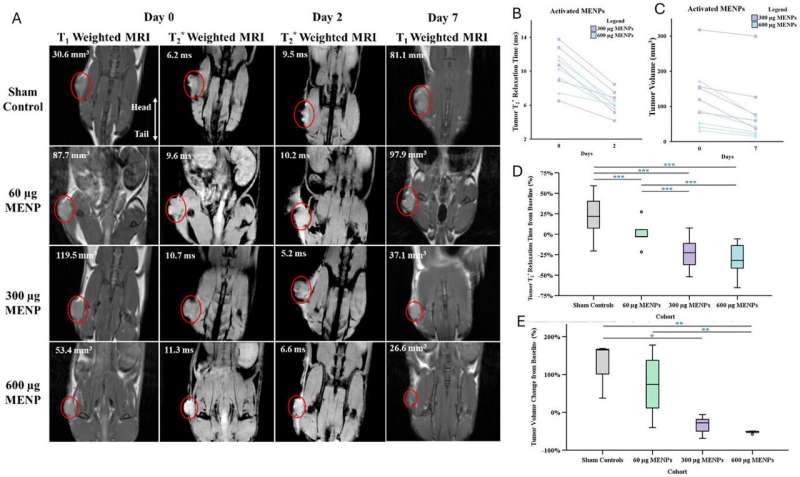
The innovative therapy utilizes tiny, wirelessly controlled particles activated by magnetic fields. In the study, a single intravenous dose of MENPs was administered, leading to a remarkable reduction in tumor size—shrinking to one-third of their original size and disappearing entirely in one-third of the treated models. The treatment also more than doubled the survival time of subjects, all while sparing healthy organs from damage.
Unlike traditional treatments such as chemotherapy and surgery, this method does not rely on drugs, heat, or invasive procedures. Instead, MENPs are injected into the bloodstream and guided to tumor sites using a small magnet. Once near the tumor, the nanoparticles are activated by the magnetic field of a standard MRI scanner. Upon activation, they generate localized electric fields that disrupt the membranes of cancer cells, inducing natural cell death without harming surrounding healthy tissues.
Advancements in Targeted Cancer Therapy
“This study brings us one step closer to connecting to the human body wirelessly to help it heal in real time,” said **Sakhrat Khizroev**, Ph.D., a professor in the College of Engineering and co-senior author of the study. He expressed hopes that this research could herald a new era in medicine, where technology can precisely target diseases previously deemed untreatable.
The research demonstrated that MENPs can be delivered directly to pancreatic tumors, with MRI scans confirming reduced tumor sizes and clear imaging signals. This dual capability presents MENPs as a powerful tool in theranostic oncology, which combines diagnostics and therapeutic response in real-time. **John Michael Bryant**, M.D., a physician-scientist at Sylvester and co-senior author, noted that the approach positions itself at the intersection of engineering, physics, and medicine, paving the way for safer and more personalized cancer care.
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal forms of cancer, with a five-year survival rate below **10%**. It is projected to become the second leading cause of cancer-related deaths in the United States by **2030**. Traditional treatment methods often inflict collateral damage on healthy tissue, while newer treatments like immunotherapy have yielded limited success.
Future Implications and Clinical Trials
The challenge of controlling electric fields to influence cancer cell growth inside the body has long hampered treatment advancements. The findings from this study suggest that MENP therapy may provide a safer, more precise alternative for patients. **Liang**, Ph.D., co-founder of Cellular Nanomed, Inc., emphasized that if this technology is successfully translated to human applications, it could revolutionize treatment approaches for pancreatic and other solid tumors.
While the current research was conducted in preclinical models, the authors believe it lays the groundwork for future clinical trials. The potential of magnetoelectric nanotherapy could lead to a new generation of wireless nanomedicine, offering hope to those facing one of the most challenging cancers.
This innovative approach minimizes side effects by eliminating the need for pharmaceutical drugs or biological reagents, which could also open avenues for treating other difficult-to-manage diseases. The study signifies a notable advancement in cancer treatment, demonstrating how emerging technologies can intersect with medical science to enhance patient outcomes.
For more information, refer to the study: John Michael Bryant et al, “Magnetoelectric Nanotherapy Achieves Complete Tumor Ablation and Prolonged Survival in Pancreatic Cancer Murine Models,” **Advanced Science** (2025). DOI: 10.1002/advs.202517228.