A team of researchers from Tohoku University has unveiled a groundbreaking method for transforming propylene into valuable industrial chemicals. This innovative process employs a low-cost material, lead dioxide (PbO2), and highlights the active role of oxygen atoms within the catalyst itself. Their findings, published on October 7, 2025, in the journal Catalysis Science & Technology, promise to enhance sustainability and affordability in the production of essential materials, including plastics, clothing fibers, and insulation foams.
Traditionally, the oxidation of propylene relies on expensive noble metals such as platinum and palladium. According to Hao Li, a professor at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) who led the study, the extraction of these metals has a considerable environmental impact. Additionally, existing industrial processes typically utilize hazardous oxidants like chlorine or peroxides, leading to significant safety and waste disposal challenges.
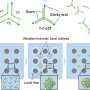
In response to these concerns, Li and his team sought a safer and greener alternative. Their research demonstrates that using lead dioxide as a catalyst, powered by electricity, enables effective catalysis of propylene oxidation. Rather than relying on external oxidants, the oxygen needed for the reaction is sourced directly from the crystal structure of the PbO2 catalyst. This process mimics a rechargeable battery, wherein the catalyst “lends” oxygen atoms to facilitate the reaction and subsequently “recharges” by extracting fresh oxygen from water present in the system.
Revolutionizing Chemical Processes
The team utilized advanced in-situ techniques to confirm this unique mechanism. Through electrochemical attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy, they identified crucial reaction intermediates forming on the catalyst’s surface. Simultaneously, differential electrochemical mass spectrometry (DEMS) provided compelling evidence of the active participation of lattice oxygen in the oxidation process.
“Our goal was to understand how non-noble metals can perform the same chemistry as noble metals but in a more sustainable manner,” Li explained. “By demonstrating that lattice oxygen plays an active role, we have opened new avenues for designing catalysts that are efficient and environmentally friendly.”
This research not only validates a novel reaction mechanism but also confirms theoretical predictions that scientists have long aspired to validate experimentally. By combining cutting-edge techniques with meticulous control of reaction conditions, the team elucidated how oxygen vacancies and lattice oxygen interact during electrochemical oxidation.
Looking ahead, the researchers intend to refine their catalyst design by modifying the electronic structure of lead dioxide. They plan to experiment with doping and oxygen-vacancy engineering to examine how varying metals and vacancy levels impact the efficiency and selectivity of the reaction.
All experimental and computational data from this study will be accessible via the Digital Catalysis Platform, a publicly available database developed by the Hao Li Lab to foster open scientific collaboration and enhance catalyst design worldwide.
The article titled “Probing the Reactivity of In-Situ Formed Oxygen Vacancies of Non-Noble Lead Oxides for Anodic Propylene Oxidation” features contributions from multiple researchers, including Jia Ge, Tian-Yi Wang, and others. The implications of this research extend beyond academic interest, offering potential pathways to greener industrial practices and a reduction in hazardous chemical use.