Research from Science Tokyo has revealed significant insights into nephronophthisis, a genetic kidney disorder linked to the deficiency of the NPHP1 gene. Utilizing human-induced pluripotent stem cell-derived kidney organoids, the team discovered that abnormal Hippo signaling plays a critical role in promoting fibrosis associated with the disease. Their findings indicate that targeting the Hippo signaling pathway could present a novel therapeutic approach to managing nephronophthisis.
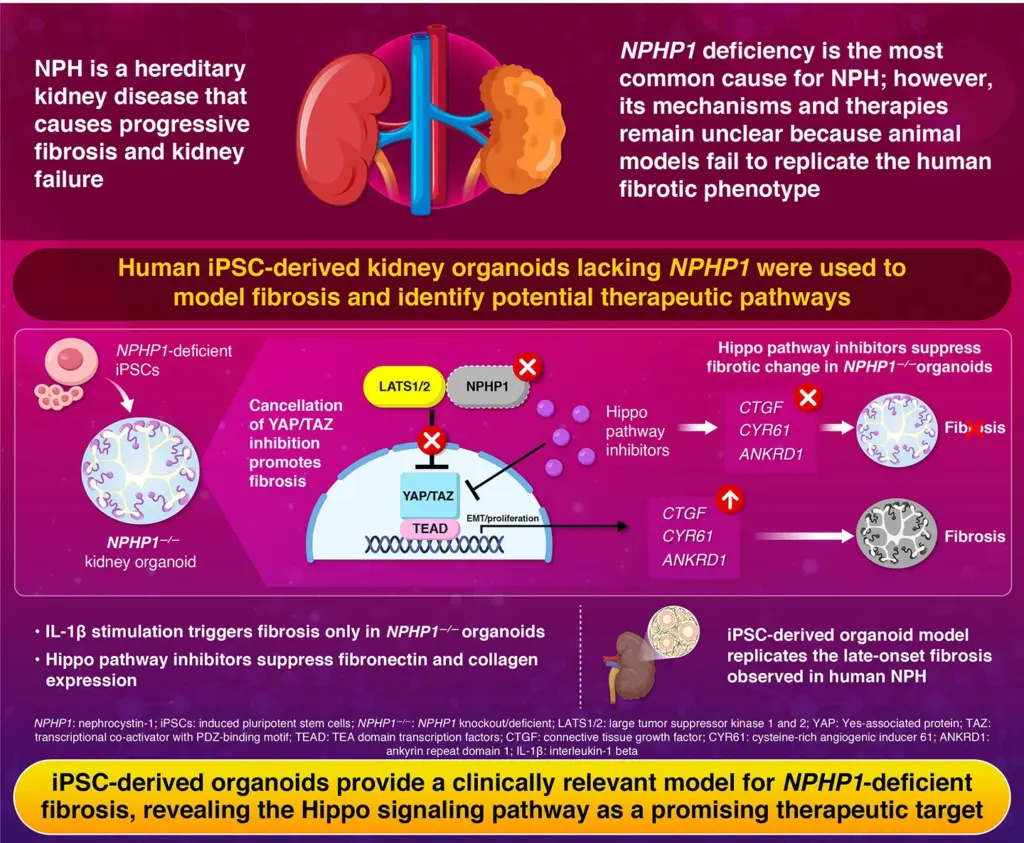
Nephronophthisis (NPHP) is a hereditary condition characterized by progressive fibrosis in the kidneys, often leading to end-stage kidney disease in children and young adults. The disorder accounts for approximately 10% of all pediatric dialysis cases. Currently, the only viable treatment option is kidney transplantation, as no effective therapies exist. The condition primarily arises from mutations or deletions in the NPHP1 gene, which is essential for the production of nephrocystin-1, a protein crucial for maintaining healthy kidney function.
Breakthrough in Understanding Disease Mechanisms
The research team, led by Associate Professor Eisei Sohara, along with Junior Associate Professor Koichiro Susa and graduate student Takefumi Suzuki, developed a human kidney organoid model using human induced pluripotent stem (iPS) cells. This innovative approach allowed them to more accurately study the disease’s mechanisms, addressing a long-standing challenge in nephronophthisis research. Their findings were published in the journal Stem Cell Research & Therapy on September 19, 2025.
“To model the NPHP1 deficiency observed in nephronophthisis, we used genome editing to remove the NPHP1 gene,” Sohara explained. The resulting NPHP1-deficient iPS cell lines were able to differentiate into three-dimensional kidney organoids that closely mimic the structure and function of human nephrons.
Upon exposure to low levels of inflammatory signals, such as interleukin-1β, these organoids exhibited pronounced fibrotic changes not seen in normal kidney organoids. Further investigation revealed that the NPHP1-deficient organoids showed an overexpression of fibrosis-related genes, indicating a significant activation of fibrosis signaling pathways.
Therapeutic Potential of Hippo Pathway Inhibition
The research identified abnormal activation of the Hippo signaling pathway in the NPHP1-deficient organoids. This pathway is essential for regulating tissue growth and repair, preventing excessive scarring by managing the activity of YAP and TAZ proteins. The absence of NPHP1 disrupts this regulation, leading to uncontrolled fibrotic signaling.
“Our findings reveal that NPHP1 interacts with components of the Hippo pathway to maintain a balance between repair and fibrosis,” Sohara noted. “When this interaction is lost, the Hippo pathway becomes overactive, driving progressive kidney damage.”
To assess the therapeutic potential of inhibiting this pathway, the team tested several Hippo pathway inhibitors. Among these, verteporfin, a drug already approved for treating macular degeneration, showed promising results. Verteporfin not only reversed fibrosis markers but also reduced the accumulation of fibrosis-related genes in the organoid models.
“Since verteporfin is already in clinical use, it offers an immediate treatment option for nephronophthisis,” Susa pointed out.
This research marks a pivotal advancement in the application of stem cell technology to develop practical therapies for rare kidney diseases. By successfully testing drugs on a human iPSC-derived NPHP model, the study demonstrates how organoid technologies can enhance disease modeling and facilitate personalized drug testing.
Looking ahead, the researchers plan to refine their kidney organoid platform to explore additional signaling pathways and evaluate further drug candidates. This work aims to pave the way for safer and more effective therapies for chronic kidney diseases, potentially transforming treatment options for patients suffering from nephronophthisis and similar conditions.