Researchers at UT Southwestern Medical Center have developed a groundbreaking genetic technique that forces cells to expel mitochondria, offering new insights into the roles these organelles play in cellular function and evolution. The study, published in the journal Cell, could pave the way for novel treatments for mitochondrial diseases such as Leigh syndrome and Kearns-Sayre syndrome.
“Our new tool allows us to study how changes in mitochondrial abundance and the mitochondrial genome affect cells and organisms,” said Dr. Jun Wu, Associate Professor of Molecular Biology at UT Southwestern. The research was co-led by Dr. Daniel Schmitz, a former graduate student in the Wu Lab, now a postdoctoral fellow at the University of California, Berkeley.
Understanding Mitochondria’s Multifaceted Roles
Mitochondria, known as the powerhouses of the cell, are organelles found in most eukaryotic organisms. They generate adenosine triphosphate (ATP), the energy currency of the cell, and are thought to have originated from prokaryotic cells that formed a symbiotic relationship with ancestral eukaryotic cells. Beyond energy production, mitochondria are involved in regulating cell death, differentiating stem cells, and transmitting molecular signals.
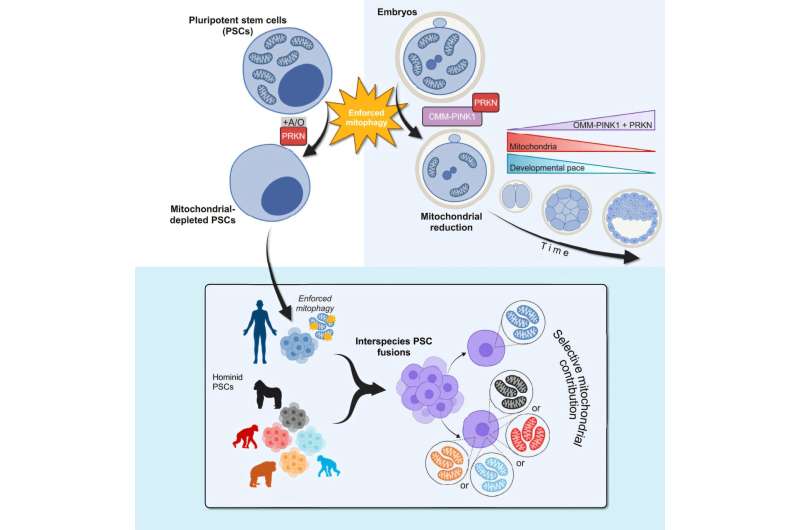
Recent studies have suggested that mitochondria communicate with nuclear DNA, but the mechanisms and effects of this “crosstalk” remain largely unknown. To explore these interactions, Dr. Wu and his team utilized a cellular pathway known as mitophagy, which cells typically use to dispose of old or damaged mitochondria. Through genetic engineering, they induced “enforced mitophagy,” compelling cells to degrade all mitochondria.
Implications for Stem Cell Research and Beyond
The researchers applied this technique to human pluripotent stem cells (hPSCs), revealing that although the cells ceased dividing, they survived for up to five days without mitochondria. This finding was consistent across different types of mouse stem cells and hPSCs with pathogenic mitochondrial DNA mutations, suggesting the method’s broad applicability.
By analyzing the nuclear gene expression of mitochondria-depleted hPSCs, the team discovered that 788 genes became less active while 1,696 genes became more active. Despite the absence of mitochondria, the cells retained their ability to differentiate into other cell types, with nuclear-encoded proteins compensating for the missing organelles’ functions.
788 genes became less active, while 1,696 genes became more active in mitochondria-depleted hPSCs.
Exploring Evolutionary and Developmental Insights
To further investigate mitochondrial-nuclear interactions, the researchers created “composite” cells by fusing hPSCs with pluripotent stem cells from humans’ closest primate relatives. These cells selectively retained human mitochondria, allowing the team to study mitochondrial interchangeability across species.
Interestingly, the genes that differed in activity between cells with human and non-human mitochondria were primarily linked to brain development and neurological diseases. This suggests that mitochondria may influence the neurological differences between humans and primates, though further research is needed to confirm these findings.
In another experiment, the team reduced mitochondrial content in mouse embryos using enforced mitophagy. Embryos missing over 65% of their mitochondria failed to implant, while those with a third of their mitochondria missing showed delayed development but eventually caught up to normal levels.
“These results serve as starting points for new lines of research into the different roles mitochondria play in cellular function, tissues and organ development, aging, and species evolution,” the researchers stated.
Future Directions and Implications
The findings from this study open new avenues for understanding the complex roles of mitochondria in cellular processes and evolution. The research team plans to continue using enforced mitophagy to explore these organelles’ functions further, potentially leading to breakthroughs in treating mitochondrial diseases.
Contributors to this study include Dr. Peter Ly, Dr. Daiji Okamura, Dr. Seiya Oura, Dr. Leijie Li, Dr. Yi Ding, Dr. Rashmi Dahiya, Emily Ballard, and Dr. Masahiro Sakurai. The study’s publication in Cell underscores its significance in advancing our understanding of mitochondrial biology.







