Recent research from Sanford Burnham Prebys has unveiled new insights into how the three-dimensional (3D) structure of the genome influences gene activity. Published on June 27, 2025, in the journal Genome Biology, the study highlights the significance of chromatin’s spatial organization in regulating gene expression and its potential links to various diseases.
Traditionally, biology education has presented the human genome and its DNA in a linear format. This simplification overlooks the complex structure of DNA, which, when tightly packed in the nucleus of cells, forms loops and clumps. The genome stretches over six feet and is coiled around proteins known as histones, creating a structure referred to as chromatin. While this arrangement may appear chaotic, it plays a crucial role in bringing certain genomic regions into closer contact while isolating others.
Research indicates that disruptions in this 3D structure are linked to several serious health conditions, including developmental disorders and various cancers. Approximately 12% of genomic regions in breast cancer cells have been found to exhibit chromatin structure anomalies. Furthermore, other structural irregularities have been implicated in conditions such as T-cell acute lymphoblastic leukemia.
Understanding the Role of Topologically Associating Domains
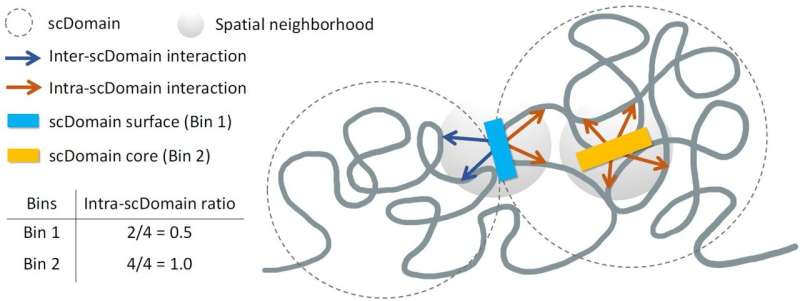
The research team, led by Kelly Yichen Li, Ph.D., a postdoctoral associate at Sanford Burnham Prebys, hypothesized that the 3D configuration of genomic regions affects gene regulation. They identified structures known as topologically associating domains (TADs), which allow parts of the genome within these domains to interact more frequently with each other, while remaining isolated from external regions.
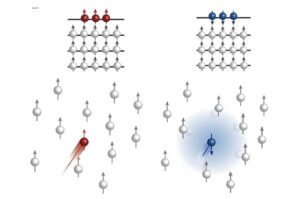
In their experiments, the researchers took multiple images of chromatin for spatial mapping and observed that TAD-like regions in individual cells often formed globular shapes. Describing these regions, Yuk-Lap (Kevin) Yip, Ph.D., interim director of the Center for Data Sciences at Sanford Burnham Prebys, noted that these structures resembled potatoes, with varying sizes and surface characteristics.
This analogy helped the team predict that regions closer to the surface of these chromatin clumps would be more active, benefiting from exposure to biochemical signals present in the cell nucleus. Conversely, genes buried deeper within these structures might find it more challenging to receive such signals.
To quantify this concept, the researchers developed a method to measure a genomic region’s proximity to the center of a chromatin clump, which they referred to as the “coreness” of the genomic region. Their findings showed that surface regions exhibited greater activity compared to those located nearer to the core.
Future Directions for Research
Yip emphasized the growing availability of data that can be analyzed using their new metric, which holds promise for studying how coreness relates to gene activity and disease across different cell types. The team plans to collaborate further with the lab of Pier Lorenzo Puri, MD, to explore the implications of 3D genome structure on muscle stem cell development and the progression of muscular dystrophy.
This research not only enhances our understanding of gene regulation but also opens new avenues for investigating the genetic underpinnings of diseases. The insights gained from studying the intricate 3D architecture of the genome could lead to innovative approaches for diagnosis and treatment in the future.
More information on the study can be found in the publication: Kelly Yichen Li et al, Regulatory roles of three-dimensional structures of chromatin domains, Genome Biology (2025). DOI: 10.1186/s13059-025-03659-7.