Significant advancements in understanding gene regulation have emerged from a recent study by researchers at Sanford Burnham Prebys, revealing how the three-dimensional (3D) structure of the genome influences gene activity. The findings, published in Genome Biology on June 27, 2025, highlight the importance of chromatin’s shape—often overlooked in traditional biological teachings focused on linear DNA presentations.
The human genome, which comprises approximately six feet of DNA, is compacted within the nucleus of cells through a complex structure known as chromatin. This arrangement not only allows the DNA to fit but also creates various loops and clusters that play a critical role in gene regulation. The study indicates that issues with chromatin structure can lead to several diseases, including developmental disorders and types of cancer.
According to the researchers, about 12% of genomic regions in breast cancer cells exhibit chromatin structural problems, while similar abnormalities are implicated in T-cell acute lymphoblastic leukemia. The research team, led by Kelly Yichen Li, Ph.D., a postdoctoral associate at Sanford Burnham Prebys, posits that the 3D configuration of DNA regions significantly affects gene regulation.
Understanding Topologically Associating Domains
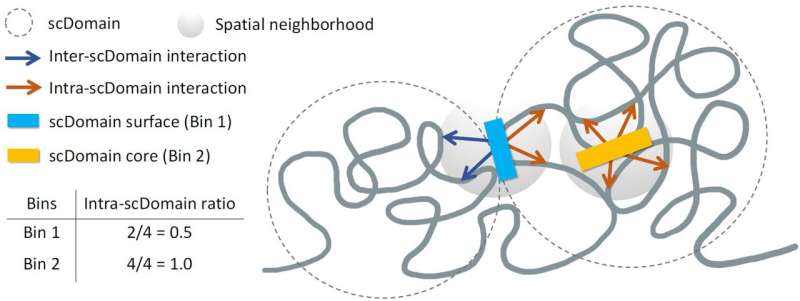
In their investigation, the team focused on what are known as topologically associating domains (TADs). These regions of the genome interact more frequently with each other while remaining insulated from surrounding areas. “Parts of the genome within these domains can interact more frequently with each other,” Li noted, emphasizing their significance in the overall genomic framework.
During their spatial mapping experiments, the researchers observed that TAD-like regions often assumed a globular form, characterized by irregularities reminiscent of a selection of potatoes. This unique shape suggested that the positioning of genomic regions within these domains could influence nearby gene functions.
Yuk-Lap (Kevin) Yip, Ph.D., a professor and interim director of the Center for Data Sciences at Sanford Burnham Prebys, explained, “If you picture these clumps of chromatin fiber being roughly in the shape of a potato, we predicted that regions of the genome closer to the surface are more active due to exposure to nearby biochemical signals in the cell nucleus.”
The researchers likened the protective role of a potato’s skin to the challenges faced by signals promoting gene expression when reaching regions buried deep within the chromatin structure. To quantify this relationship, they developed a metric to measure a genomic region’s proximity to the core of a chromatin clump, allowing them to distinguish between surface and core regions.
Implications for Future Research
The study revealed that genomic regions on the surface of chromatin domains exhibited higher activity levels compared to those situated towards the core. “This measure also allowed us to define the surface and core, and we went on to show that surface regions are more active than core regions,” Li explained, highlighting the potential applications of their findings.
Yip expressed enthusiasm about the volume of data available for future studies, stating, “There is a lot of potential to study how coreness links to gene activity and disease in different cell types.” The researchers plan to collaborate with Pier Lorenzo Puri, MD, to further explore how the 3D structure of the genome impacts muscle stem cell development and the progression of muscular dystrophy.
This groundbreaking research not only enhances our understanding of gene regulation but also opens new avenues for investigating how chromatin structure may contribute to various diseases, paving the way for potential therapeutic strategies in the future.