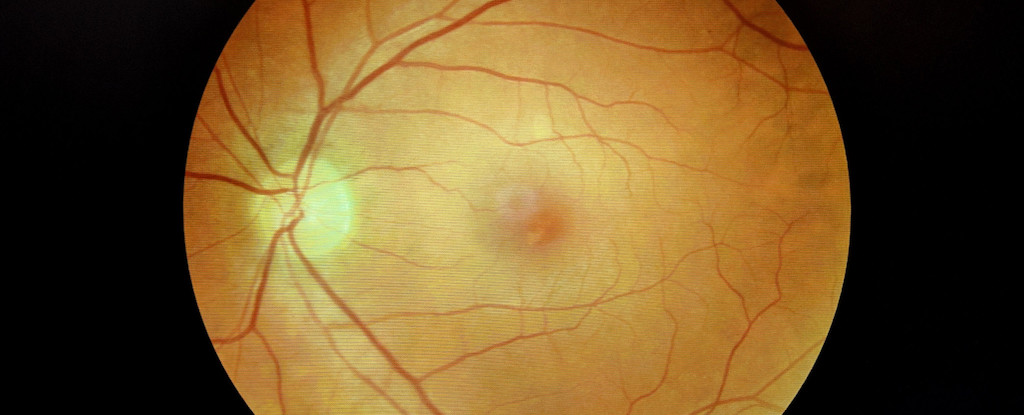
A recent study led by researchers at Yale University has revealed that COVID-19 may cause the accumulation of proteins resembling those found in Alzheimer’s disease, not only in the brain but also in the eyes. This finding could potentially explain the phenomenon known as “brain fog,” a term commonly used to describe cognitive difficulties reported by many individuals following COVID-19 infection.
The research, published in Science Advances, investigates the connection between COVID-19 and cognitive impairment. Senior author Dr. Brian Hafler, an ophthalmologist at the Yale School of Medicine, noted, “There is growing evidence linking COVID-19 and brain fog, a commonly reported symptom following infection.” The study highlights how the virus, specifically the SARS-CoV-2 spike protein, can induce the accumulation of amyloid beta in the central nervous system, which plays a significant role in Alzheimer’s pathology.
The retina, a part of the central nervous system, offers a unique opportunity for researchers to examine the effects of COVID-19. Previous studies have indicated that amyloid beta builds up in the retinas and brains of Alzheimer’s patients, suggesting that retinal assessments could be a useful tool for diagnosing and monitoring the disease.
To explore this further, the Yale researchers utilized postmortem human retinal tissue and developed retinal organoids—three-dimensional models derived from human stem cells. They focused on two proteins, neuropilin-1 (NRP1) and angiotensin-converting enzyme 2 (ACE2), which have been identified as potential entry points for SARS-CoV-2 into neurons. The presence of NRP1 in the retinal tissue of individuals who contracted COVID-19 raises concerns about the virus’s ability to access the eyes.
The findings revealed that individuals without a history of dementia exhibited increased levels of amyloid beta if they had contracted COVID-19, mimicking Alzheimer’s-like retinal tissue. Additionally, the researchers observed that exposure to the spike protein of SARS-CoV-2 resulted in heightened amyloid beta levels in retinal organoids. When they introduced an NRP1 inhibitor, they were able to reduce the increase in amyloid beta, suggesting a potential therapeutic target to mitigate neurological complications associated with COVID-19.
Dr. Hafler stated, “Mechanistically, the involvement of NRP1 in amyloid beta aggregation gives a specific molecular target for future investigation.” This study not only sheds light on the cognitive challenges following COVID-19 but also supports the hypothesis that amyloid beta may serve as a protective mechanism for the brain against viral infections.
The implications of this research extend beyond COVID-19, as the authors suggest that other viral infections could similarly trigger amyloid beta accumulation. Ongoing clinical studies are being conducted to determine whether COVID-19 increases the long-term risk of Alzheimer’s disease.
“Our ultimate goal is to prevent long-term neurological effects of COVID-19 and explore NRP1 inhibitors and other modulators of virus-host interactions as potential therapeutics for preventing viral-induced amyloid pathology and Alzheimer’s disease,” Dr. Hafler concluded.
As the world continues to grapple with the effects of COVID-19, understanding its impact on brain health remains paramount. This study opens new avenues for research and potential interventions aimed at addressing the cognitive repercussions of the virus.