Researchers have achieved a groundbreaking milestone by successfully filming the exact moment a human embryo implants itself into the uterine wall. This first-of-its-kind observation offers new insights into a critical stage of human development, which could significantly enhance fertility treatments. The study, conducted by a team at the Barcelona Institute of Science and Technology (BIST), utilized advanced time-lapse imaging to unveil the complexities of embryo implantation, a process that remains largely elusive in the field of reproductive science.
Implantation typically occurs five to six days after fertilization, when a cluster of 100 to 200 cells, known as a blastocyst, seeks to establish itself within the mother’s uterus. Until now, scientists have only been able to capture static images of this vital process. With the new technology developed by the research team, they can observe the dynamic actions of embryos as they penetrate a collagen-based matrix designed to mimic the uterine environment.
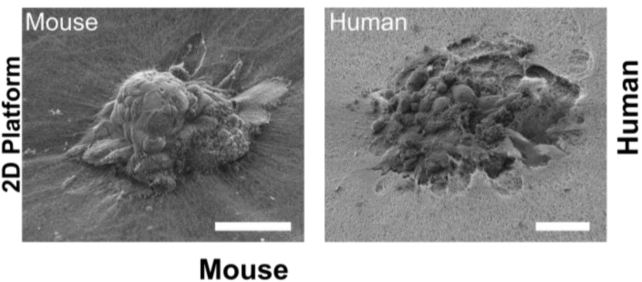
Samuel Ojosnegros, the senior author of the study, explained, “For the first time, we’ve been able to watch human embryo implantation unfold dynamically. We’ve opened a window into a stage of development that was previously hidden.” The findings, published in Science Advances, highlight how the embryos not only attach to the surface of the matrix but also actively invade it, a behavior not seen in mouse embryos, which typically only penetrate superficially.
Understanding the Mechanisms of Implantation
The research team created a specialized platform that replicates the structural conditions and nutrients necessary for embryos to implant successfully. This innovation addresses the crucial fact that approximately 60 percent of failed pregnancies occur during implantation or shortly thereafter. Ojosnegros noted the importance of this stage, stating, “This means that mouse studies can only take us so far in understanding human implantation.”
The time-lapse imaging revealed that human embryos exert significant mechanical force to navigate through the collagen matrix. This invasive technique allows them to fully envelop themselves in the supportive structure, which is essential for further growth. By observing these dynamics, researchers can better understand the factors that influence successful implantation.
In the lab, the embryos were placed on a flat gel surface or within droplets of the collagen matrix. This approach offered two perspectives: in the first, embryos were seen binding to the matrix; in the second, they appeared to “pull” collagen fibers towards their center, effectively remodeling their environment. Lead author Amélie Luise Godeau and her team suggest that this behavior may indicate a connection between the embryo and the maternal tissues, although the exact implications of these interactions require further study.
Potential Applications for Fertility Treatments
While the collagen-based matrix used in the experiments does not include human uterine cells, it serves as a powerful tool for experimentation. Researchers can modify its composition to explore how embryos respond to various environmental factors or compounds that could enhance implantation rates. Ojosnegros highlighted the commercial potential of their research, stating that through their spin-off company, Serabiotics, in collaboration with pharmaceutical company Grifols, they have developed a protein supplement aimed at improving implantation success in clinical settings.
The insights gained from this research could pave the way for improved fertility treatments, offering hope to those facing challenges in conceiving. By delving into the intricacies of human embryo implantation, scientists hope to unlock new strategies that could lead to higher success rates in assisted reproductive technologies.
As this groundbreaking research continues to unfold, the implications for our understanding of human development and reproductive health are profound. The ability to visualize and investigate the early stages of life could not only enhance scientific knowledge but also foster advancements in fertility treatments that may benefit countless families in the future.