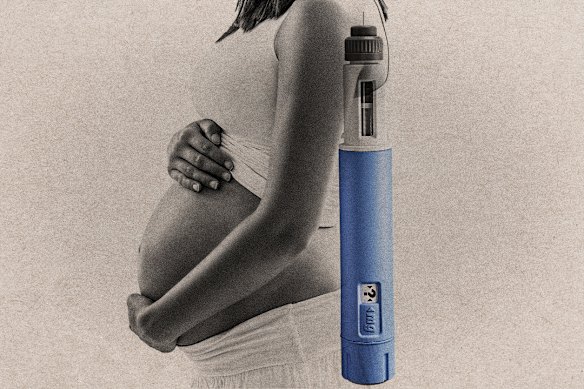
Concerns are growing regarding the use of weight-loss injections and their potential impact on contraceptive effectiveness, as numerous women report unplanned pregnancies while using these medications. Australia’s medicines regulator has indicated that it cannot dismiss the possibility that GLP-1 inhibitors, commonly prescribed for type 2 diabetes and weight loss, may interfere with oral contraceptives.
Women using these injections are advised to discontinue them at least one to two months prior to attempting to conceive due to worries about their effects on fetal development. Reports of unintended pregnancies are rising, leading to the emergence of the term “Ozempic babies,” as many women experience improved fertility alongside the weight loss effects of the drugs.
MotherSafe, which offers counseling services for women in New South Wales (NSW), has reported receiving 130 calls this year pertaining to GLP-1 exposure during pregnancy. Dr. Debra Kennedy, the director of MotherSafe, emphasized the importance of planning pregnancies. She stated, “It’s important that people plan pregnancies and are aware that these drugs may improve their chances of falling pregnant.”
In Victoria, Monash Health has assisted at least 12 women who became pregnant while undergoing treatment with weight-loss injections over the past year. Additionally, inquiries about semaglutide exposure, the active ingredient in Ozempic, have surged at the Royal Women’s Hospital, according to a spokesperson for the institution.
New research published in the Medical Journal of Australia further substantiates these concerns. The study found that 2 percent of women of reproductive age taking GLP-1s, translating to 232 women, became pregnant within six months of starting the treatment. The rate increased to 4 percent among women aged 25 to 34, who are often in their peak reproductive years.
Lead author Luke Grzeskowiak, a pharmacist and researcher associated with Flinders University, noted that animal studies have linked GLP-1 exposure during pregnancy to adverse effects, including reduced fetal growth and slowed bone development.
As the number of women using these medications rises, public health officials are urging increased awareness of the potential implications for reproductive health. The intersection of weight loss and fertility is creating a complex landscape that necessitates careful consideration for women who are or may become pregnant while using these drugs.
With reports of unplanned pregnancies continuing to emerge, it remains critical for healthcare providers to communicate effectively with patients about the risks associated with GLP-1s. This situation underscores the need for women to have comprehensive discussions with their healthcare providers regarding family planning and the medications they are using.