A team of physicists from the University of Amsterdam has achieved a significant breakthrough in measuring the g-factor of strontium atoms, an advancement that could enhance the precision of atomic clocks and quantum computing applications. Using nearby rubidium atoms as a calibration tool, the researchers determined the g-factor of the 87 Sr isotope with unprecedented accuracy, as detailed in their recent publication in Physical Review Letters on November 4, 2025.
Strontium, while not widely recognized outside scientific circles, is a member of the alkaline earth metals and is known for its unique properties that make it a prime candidate for modern technological applications. The 87 Sr isotope is particularly notable due to its nuclear spin, which transforms it into a fermion—a particle that behaves differently than its bosonic counterparts. This characteristic is crucial for its function in next-generation optical atomic clocks and quantum computers.
The importance of 87 Sr lies in its ability to emit and absorb light at highly precise frequencies. The optimal frequency for this isotope corresponds to a wavelength of 698 nanometers, producing a clear red light. Unlike the bosonic isotopes, which have zero total spin and thus cannot effectively transition states, the fermionic nature of 87 Sr allows for the necessary transitions to occur, enabling the development of highly accurate atomic clocks.
Enhancing Precision Through Innovative Techniques
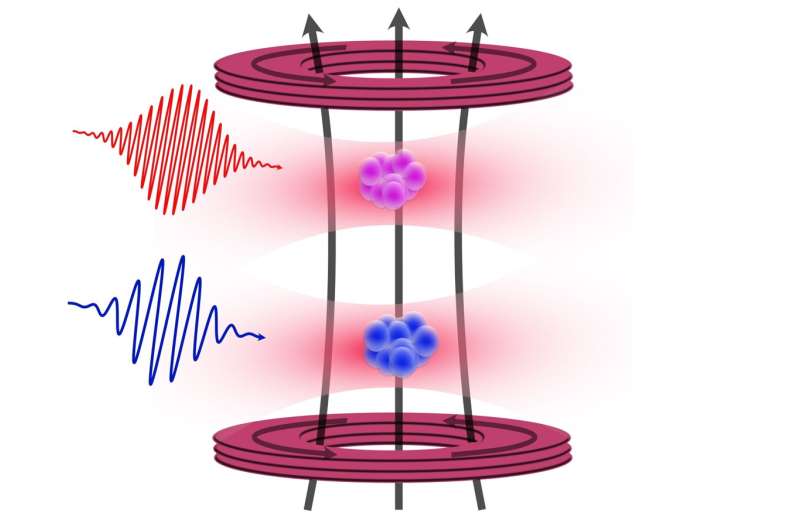
The research team, led by physicist Premjith Thekkeppatt, initially aimed to create rubidium-strontium molecules. However, the challenges they faced prompted them to explore the interactions between these two elements without direct overlap. By employing optical trapping, they successfully positioned both 87 Sr and rubidium atoms in close proximity, setting the stage for further investigation.
Utilizing a technique known as nuclear magnetic resonance, the team measured the energy splitting in the strontium nucleus, which directly correlates with the strength of its nuclear magnet. This process involved leveraging the well-characterized properties of rubidium to calibrate the magnetic field strength accurately. The outcome was a remarkable one-hundredfold improvement in the precision of the g-factor measurement for 87 Sr.
The implications of this enhanced measurement are significant, not only for atomic clock technology but also for quantum computing. The Zeeman effect, first described by Nobel laureate Pieter Zeeman in 1896, plays a pivotal role in determining how the energy levels of atoms split in a magnetic field. The enhanced precision of the g-factor will allow researchers to refine the operation of optical clocks and explore new quantum computing possibilities.
Implications for Future Research
The results from this research establish a new benchmark for atomic structure calculations and precision measurements. Thekkeppatt commented on the importance of their findings, stating, “Our results form a new challenging benchmark for atomic structure calculations. We have shown that this new method works very well for precision measurements, and the demonstrated methods will inspire extension to further atomic species and states relevant for all sorts of applications.”
The research not only paves the way for advancements in atomic clocks and quantum computing but also sets the stage for future studies involving other atomic species. As scientists continue to explore the potential of strontium and its properties, this groundbreaking work is expected to contribute significantly to both theoretical understanding and practical applications in the field of quantum technology.
For further details, the complete study is available in Physical Review Letters.