A newly discovered bacterial enzyme shows promise for the sustainable production of ethylene, a key ingredient in plastics. Researchers from the Max Planck Institute for Terrestrial Microbiology have identified the enzyme methylthioalkane reductase, produced by the bacterium Rhodospirillum rubrum, which operates in oxygen-free conditions without emitting carbon dioxide.
The current global demand for plastics relies heavily on ethylene derived from fossil fuels, making the search for renewable production methods increasingly urgent. Traditional processes not only require energy-rich substrates but also generate carbon dioxide as a by-product. The identification of this specific enzyme has sparked interest in its potential applications, although challenges in studying its properties have persisted.
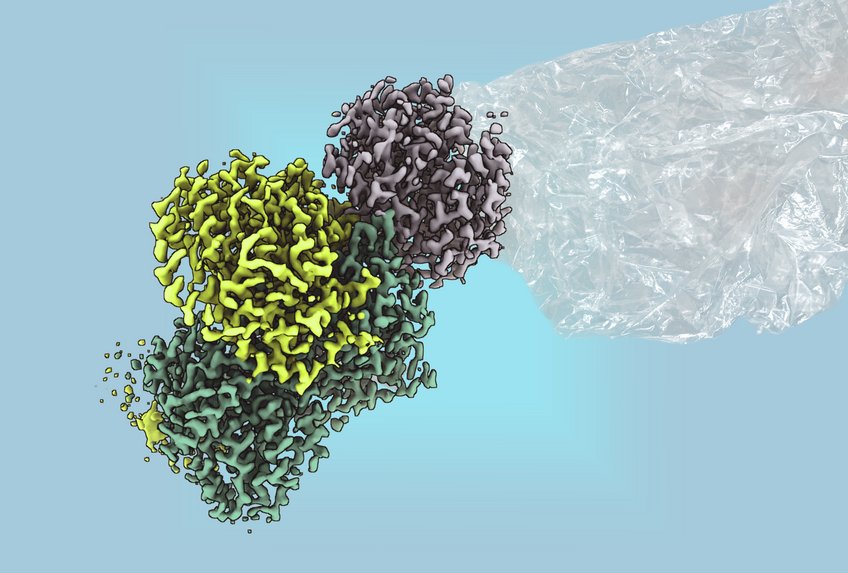
Research led by Johannes Rebelein has made significant strides in understanding this enzyme. Collaborating with RPTU Kaiserslautern, the team succeeded in purifying the enzyme and elucidating its structure. Their findings reveal that the enzyme’s catalytic activity is driven by intricate iron-sulfur clusters, previously associated only with nitrogenases, some of the oldest enzymes known to science.
“It is astonishing that methylthioalkane reductase is the first non-nitrogenase enzyme discovered to contain these complex metal clusters,” said Ana Lago-Maciel, a doctoral student and the study’s first author. The presence of these clusters indicates a biochemical pathway that could lead to the sustainable production of various hydrocarbons, including ethylene, ethane, and methane.
The research also highlights the structural and functional differences between methylthioalkane reductase and nitrogenases. While nitrogenases play a critical role in the reduction of atmospheric nitrogen, the new enzyme expands our understanding of how metal clusters can influence reactivity within different contexts.
“Our study provides the in-depth structural knowledge necessary to harness these reductases for biotechnological applications,” Rebelein added. This knowledge could lead to tailored production processes that meet the growing demand for sustainable materials.
The implications of this research extend beyond the immediate benefits of renewable plastic production. It offers insights into the evolutionary history of these significant biological catalysts, suggesting that structurally similar enzymes may have been utilizing these iron-sulfur clusters for reductive catalysis long before nitrogenases emerged.
Overall, the findings from the Max Planck Institute highlight a significant shift in the understanding of microbial processes and their potential for addressing contemporary environmental challenges. With further research and development, this enzymatic approach could play a pivotal role in creating a more sustainable future for the plastics industry.






