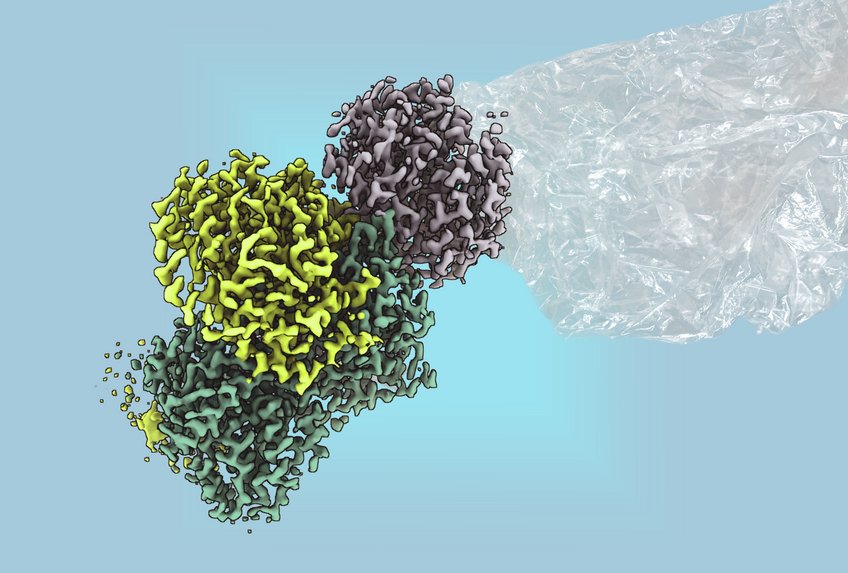
A recent breakthrough in microbial research could revolutionize the sustainable production of ethylene, an essential component in plastic manufacturing. Researchers at the Max Planck Institute for Terrestrial Microbiology, in collaboration with RPTU Kaiserslautern, have isolated and characterized an enzyme known as methylthio-alkane reductase from the bacterium Rhodospirillum rubrum. This enzyme facilitates the production of ethylene without releasing carbon dioxide, addressing significant environmental concerns associated with conventional fossil fuel-based methods.
Traditionally, ethylene is derived from fossil fuels, a process that contributes to greenhouse gas emissions. The need for renewable alternatives has become increasingly urgent. While some naturally occurring enzymes can produce ethylene, they typically require energy-rich substrates and emit CO2 as a by-product. The discovery of the methylthio-alkane reductase enzyme sparked excitement within the scientific community due to its ability to produce ethylene in oxygen-free environments without the carbon emissions typically associated with such processes.
The challenges of studying this enzyme arose from its sensitivity to oxygen, which limited previous research to cell cultures. Many questions remained about its functionality and potential applications. Now, under the guidance of lead researcher Johannes Rebelein, the team successfully purified the enzyme and elucidated its structure, providing essential insights into its catalytic mechanisms.
Significant Discoveries Unveiled
The research revealed that the enzyme is powered by large, complex iron-sulfur clusters, previously believed to exist only in nitrogenases—some of the oldest enzymes known to humanity. According to Ana Lago-Maciel, a doctoral student and the study’s first author, “The reaction is driven by these complex clusters, which are critical to the enzyme’s function.” This discovery marks the methylthio-alkane reductase as the first non-nitrogenase enzyme identified to contain these significant metal clusters.
Nitrogenases play a crucial role in reducing atmospheric nitrogen, allowing for its incorporation into vital biomolecules like DNA and proteins. The structural complexity of their iron-sulfur clusters has long been acknowledged as a key feature in biological systems. The findings from this study not only deepen the understanding of this enzyme’s capabilities but also suggest that similar enzymes may have existed long before nitrogenases evolved.
Implications for Biotechnology and Environmental Sustainability
The versatility of methylthio-alkane reductase extends beyond ethylene production. The enzyme can sustainably generate a variety of hydrocarbons, including ethane and methane. Rebelein emphasizes the broader implications of their findings, stating, “Our study provides the in-depth structural knowledge needed to harness these reductases biotechnologically and tailor their product spectrum to meet our needs.”
The research showcases significant potential for developing renewable processes that can contribute to reducing reliance on fossil fuels. Understanding the enzymatic mechanisms at play may also offer insights into the evolutionary history of complex biological systems, particularly regarding the utilization of metal clusters for reductive catalysis.
This breakthrough sets the stage for further exploration into the biotechnological applications of methylthio-alkane reductase, paving the way for a more sustainable future in the production of essential materials. As the world grapples with climate change and environmental degradation, innovative solutions like these provide hope for a greener tomorrow.






