A research team at The Hong Kong University of Science and Technology (HKUST) has made a groundbreaking discovery regarding the internal transport mechanisms of proteins within cells. Led by Prof. Guo Yusong, an Associate Professor in the Division of Life Science, the team has identified new cargo proteins and vital accessory factors associated with two essential cellular transport complexes, AP-1 and AP-4. These findings, published in the Proceedings of the National Academy of Sciences (PNAS), have implications for understanding hereditary diseases linked to errors in cellular transport.
By utilizing a novel vesicle proteomics platform, the researchers have developed a comprehensive map outlining previously unknown cargo proteins and regulatory factors. The secretory pathway in cells functions similarly to a postal service, ensuring proteins reach their intended destinations to maintain proper cellular function. Malfunctions in this transport system can lead to severe physiological defects.
“For years, the field has struggled to comprehensively map the cargo repertoire of adaptor complexes like AP-1 and AP-4, whose malfunctions are directly linked to serious human conditions such as MEDNIK syndrome, X-linked intellectual disability, and AP-4 deficiency syndrome,” stated Prof. Guo. He emphasized the study’s significance in moving from a piecemeal understanding of these complexes to a more holistic view of their cargo landscape.
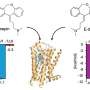
The research employs an innovative combination of vesicle reconstitution techniques and quantitative mass spectrometry-based proteomics. This allows the team to recreate transport vesicles in a controlled environment, followed by a detailed analysis of their protein composition. Collaborating with Prof. Yao Zhong-Ping from The Hong Kong Polytechnic University (PolyU), the researchers achieved significant insights into how specific cargo proteins are transported from the trans-Golgi network, a central sorting hub within the cell.
Notably, the study identified that the protein CAB45 is dependent on AP-1 for transport, while the protein ATRAP represents a novel cargo for AP-4. A critical finding addressed a long-standing question regarding how AP-4 forms transport vesicles without the widely recognized protein clathrin. The research unveiled that two cytosolic factors, WDR44 and PRRC1, are essential accessories for AP-4-mediated trafficking. When these factors were depleted, key cargo proteins like ATG9A and ATRAP were unable to exit their organelles, disrupting critical cellular processes such as autophagy.
“Our findings not only reveal new cargo clients and essential co-factors for AP-1 and AP-4 but also provide a powerful toolkit for the scientific community to dissect the mechanisms of vesicular trafficking,” added Prof. Guo. “This opens new avenues for researching the pathological mechanisms of related diseases and potentially identifying new therapeutic targets.”
The study represents a significant advancement in cell biology, with potential implications for developing therapies aimed at correcting the dysfunctions associated with various genetic diseases. The collaborative effort between HKUST and PolyU highlights the importance of interdisciplinary approaches in addressing complex biological challenges. Dr. PENG Ziqing, a postdoctoral researcher at HKUST, served as the first author of this study, showcasing the contributions of emerging scientists in this vital field of research.