A collaborative research effort between scientists from South Korea and Japan has led to the discovery of a revolutionary crystal that can “breathe” oxygen. This unique property of the crystal allows it to absorb and release oxygen at relatively low temperatures, opening new avenues for clean energy technologies and advanced electronic devices. The findings were published in the journal Nature Communications on August 15, 2025.
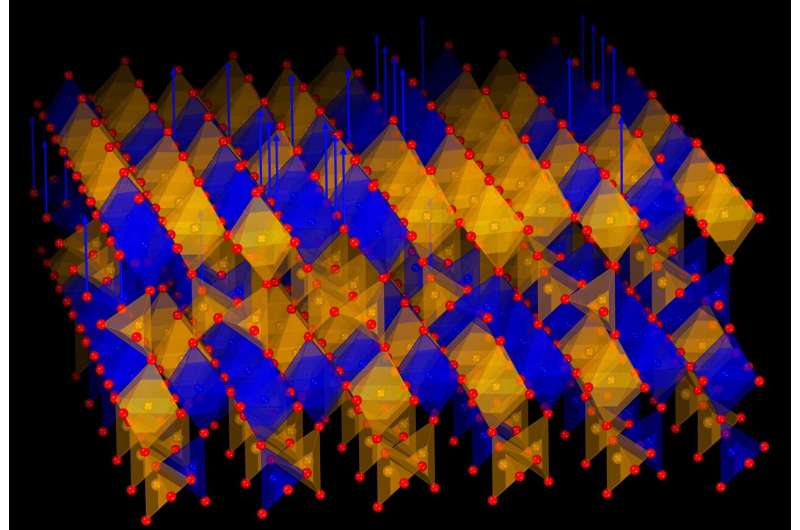
The material, a specialized metal oxide composed of strontium, iron, and cobalt, exhibits an extraordinary capability: it can release oxygen when heated in a simple gas environment and reabsorb it without deteriorating. This reversible process can occur repeatedly, making the crystal suitable for practical applications.
Significance for Clean Energy Technologies
Led by Professor Hyoungjeen Jeen from the Department of Physics at Pusan National University, and co-authored by Professor Hiromichi Ohta from the Research Institute for Electronic Science at Hokkaido University, the study highlights the potential of this material in various technologies. “It is like giving the crystal lungs; it can inhale and exhale oxygen on command,” explains Professor Jeen.
The ability to control oxygen in materials is vital for applications such as solid oxide fuel cells, which generate electricity from hydrogen with minimal emissions. Additionally, this crystal’s properties can enhance thermal transistors—devices that manage heat like electrical switches—and smart windows that adjust heat flow based on environmental conditions.
Previous materials capable of oxygen control often required extreme conditions or were too fragile for practical use. In contrast, this new crystal operates effectively under milder conditions while maintaining structural stability.
New Insights into Material Properties
Professor Jeen notes the significance of their findings, stating, “This discovery is striking in two ways: only cobalt ions are reduced, and the process leads to the formation of an entirely new but stable crystal structure.” The researchers demonstrated that the material could return to its original state when oxygen is reintroduced, confirming the complete reversibility of the process.
“This is a major step toward the realization of smart materials that can adjust themselves in real time,” says Professor Ohta. The implications of this breakthrough extend far beyond clean energy, potentially influencing electronics and environmentally friendly building materials.
The research team has laid the groundwork for further exploration into these materials, which could lead to advancements in various sectors reliant on energy efficiency and sustainable practices.
For more information on the study, refer to Joonhyuk Lee et al, “Selective reduction in epitaxial SrFe 0.5 Co 0.5 O 2.5 and its reversibility,” published in Nature Communications (2025). DOI: 10.1038/s41467-025-62612-1.