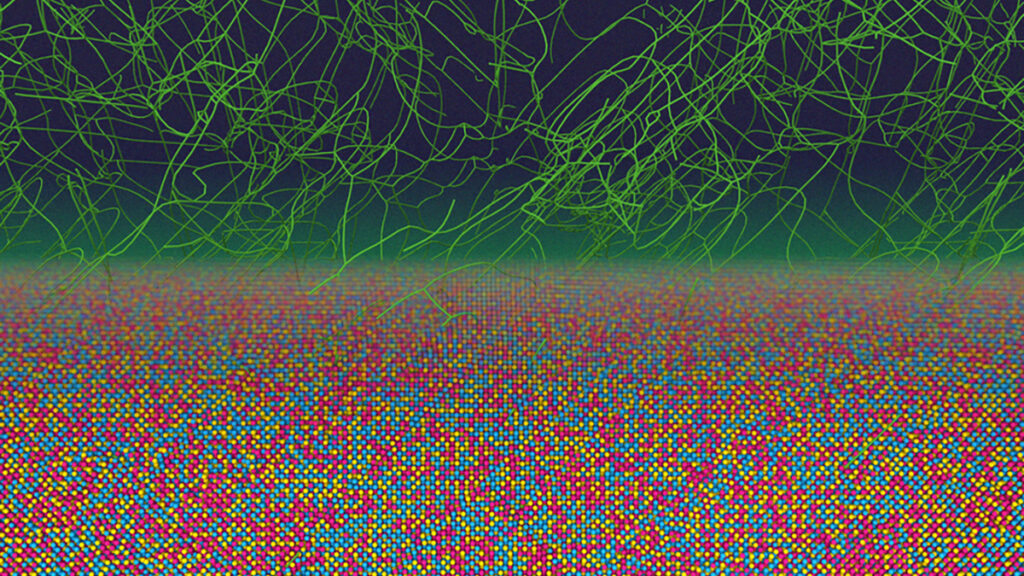
Recent research from the Massachusetts Institute of Technology (MIT) has challenged established beliefs regarding the atomic structure of metal alloys. The study reveals that unique atomic patterns persist even after intense processing, providing insights that could significantly enhance the production and properties of metals used in various applications.
Discovering Non-Random Atomic Arrangements
Traditionally, it was assumed that the atoms in metal alloys are mixed randomly during manufacturing, leading to a uniform distribution. However, this new investigation indicates that subtle, ordered patterns can survive extreme manufacturing conditions, such as rapid cooling and extensive deformation. These findings suggest a new avenue for controlling the mechanical strength, durability, and radiation tolerance of metals.
In the study, researchers utilized advanced computer simulations to analyze the behavior of millions of atoms in an alloy composed of chromium, cobalt, and nickel (CrCoNi). They observed familiar atomic patterns that were unexpectedly retained during processing. More intriguingly, they also identified novel configurations, termed “far-from-equilibrium states,” which are not typically seen in standard material behavior.
Rodrigo Freitas, a materials scientist at MIT, emphasized the significance of these findings: “This is the first paper showing these non-equilibrium states that are retained in the metal. Right now, this chemical order is not something we’re controlling for or paying attention to when we manufacture metals.” This insight has the potential to transform how engineers approach metal production.
Implications for Future Metal Manufacturing
The study revealed that defects, or dislocations, within the crystal structure of metals play a crucial role in maintaining these ordered atomic arrangements. As metals undergo heating, cooling, or stretching, these defects act like atomic-level guides, influencing how atoms move and organize themselves. Freitas noted, “These defects have chemical preferences that guide how they move. They look for low energy pathways, so given a choice between breaking chemical bonds, they tend to break the weakest bonds, and it’s not completely random.”
The implications of this research extend far beyond theoretical interest. By understanding how to manipulate these atomic patterns, manufacturers could optimize the properties of metal alloys for critical applications, including in nuclear reactors and spacecraft. The findings suggest that it is impossible to fully randomize the atomic structure of metals, regardless of processing methods. “The conclusion is: you can never completely randomize the atoms in a metal. It doesn’t matter how you process it,” Freitas stated.
Published in the journal Nature Communications, this study opens the door to innovative approaches in material science, suggesting that the atomic structure of metals can be fine-tuned in ways previously unconsidered. As researchers continue to explore these non-equilibrium states, the potential for advancements in metal manufacturing is significant.







