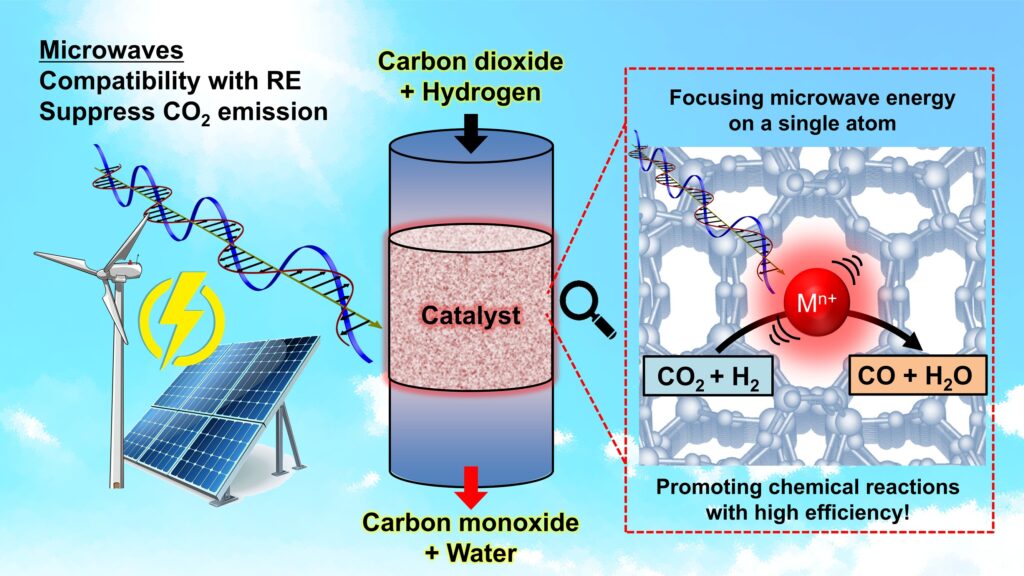
Researchers from the University of Tokyo have developed an innovative technique that significantly enhances the efficiency of chemical reactions in industrial processes. By utilizing microwaves, similar to those found in household ovens, they have achieved a method that is approximately 4.5 times more efficient than traditional heating methods.
This advancement targets the challenge of heating in chemical processes, where conventional techniques often waste energy by heating larger volumes than necessary. The new approach focuses on delivering heat precisely to the areas where it is needed, thus optimizing energy consumption. Fuminao Kishimoto, a lecturer in the Department of Chemical System Engineering at the University of Tokyo, emphasizes the importance of this research in the context of climate change and the broader goal of green transformation.
Microwave Technology Applied to Chemical Reactions
Kishimoto explains that chemical reactions typically occur at localized regions involving only a few atoms or molecules. Traditional methods, such as combustion or hot fluids, disperse thermal energy throughout the entire reactor. The team’s innovative approach instead concentrates energy on a single atomic site, akin to how a microwave oven heats food.
The researchers utilized microwaves tuned to 900 megahertz, lower than the common frequency of 2.45 gigahertz used in microwave ovens. This specific tuning is ideal for exciting the material they needed to heat, which is zeolite. Kishimoto notes, “The most challenging aspect was proving that only a single atomic active site was being heated by the microwaves.”
After four years of research at Japan’s renowned SPring-8 synchrotron radiation facility, the team developed a specialized experimental environment. They employed spongelike zeolite, allowing for controlled sizes of sponge cavities that optimize various reaction factors. Within these cavities, indium ions act as antennas, absorbing microwaves and generating heat that can be transferred to the reaction materials.
Potential Applications and Future Challenges
This innovative heating technique offers significant advantages for industrial applications, particularly in reactions requiring high temperatures. By selectively delivering heat, the researchers can lower the overall temperatures needed for processes such as water decomposition and methane conversion, both critical for producing fuel products.
Furthermore, the adaptability of the zeolite sponge allows for variations in pore size, enhancing selectivity. Smaller pores yield greater efficiency, while larger pores provide better control over reactions. The technique also holds promise for applications in carbon capture, potentially recycling CO2 during methane conversion and facilitating easier recycling of plastics.
Despite these breakthroughs, there remain challenges to scaling this technology for industrial use. The complexity and cost of material production present hurdles, along with difficulties in accurately measuring temperatures at the atomic scale. Current data rely on indirect evidence, and more precise methods are needed for validation.
Kishimoto acknowledges, “We aim to expand this concept to other important chemical reactions beyond CO2 conversion and to further optimize catalyst design to improve durability and scalability.” While the technology is still in the laboratory phase, the team anticipates pilot-scale demonstrations within the next decade. Broader industrial adoption will depend on advancements in technology and energy infrastructure.
To facilitate this progress, the researchers are seeking corporate partners for joint development initiatives. As Kishimoto concludes, “Scaling up will require further development of catalysts, reactor design, and integration with renewable power sources.” The ongoing efforts from the University of Tokyo could mark a significant step forward in the quest for energy-efficient chemical production.