New research from the Sanford Burnham Prebys Medical Discovery Institute and collaborators in Hong Kong has revealed significant insights into how the three-dimensional (3D) structure of chromatin influences gene regulation. Published on June 27, 2025, in the journal Genome Biology, the study suggests that the spatial organization of DNA within cells plays a crucial role in gene activity, impacting various diseases, including cancer.
Traditionally, the human genome has been represented in a linear format, which simplifies the understanding of DNA’s structure. However, this perspective neglects the complexity of chromatin, where DNA is packaged around proteins called histones to fit within the nucleus of a cell. The arrangement of chromatin into loops and clumps may seem chaotic but is essential for genomic function. Disruptions in this 3D structure have been linked to approximately 12% of genomic regions affected in breast cancer cells and other conditions like T-cell acute lymphoblastic leukemia.
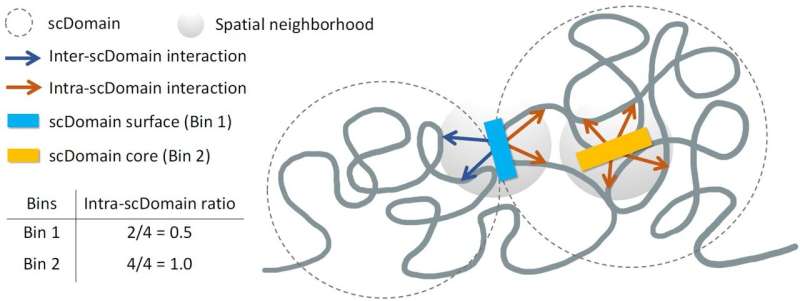
In their research, the team led by Kelly Yichen Li, Ph.D., a postdoctoral associate at Sanford Burnham Prebys, explored how the 3D shape of genomic regions affects gene regulation. “We know that many regions of the genome tend to form what are known as topologically associating domains or TADs,” Li explained. These TADs allow certain parts of the genome to interact more frequently, while isolating them from regions outside their domain.
The researchers utilized spatial mapping experiments to analyze the chromatin of individual cells. They observed that TAD-like regions consistently exhibited a globular shape, akin to a selection of potatoes in a supermarket. This discovery led them to hypothesize that regions closer to the surface of these chromatin structures are more active, as they are more accessible to biochemical signals within the cell nucleus.
Yuk-Lap (Kevin) Yip, Ph.D., who serves as a professor and interim director of the Center for Data Sciences at Sanford Burnham Prebys, highlighted the protective nature of the chromatin’s structure. “If you picture these clumps of chromatin fiber being roughly in the shape of a potato, we predicted that regions of the genome closer to the surface are more active due to exposure to nearby biochemical signals,” Yip said.
To validate their hypothesis, the researchers developed a method to measure the “coreness” of genomic regions within these chromatin domains. Li noted, “We used a metric to quantify the ‘coreness’ of a genomic region. This measure also allowed us to define the surface and core, and we went on to show that surface regions are more active than core regions.”
The findings have significant implications for understanding gene activity and its connection to diseases. “The type of data we can apply this measure to is becoming quite plentiful,” Yip added. “There is a lot of potential to study how coreness links to gene activity and disease in different cell types.”
Looking ahead, Li and Yip plan to collaborate with Pier Lorenzo Puri, MD, to further investigate how the 3D structure of the genome affects muscle stem cell development and the progression of muscular dystrophy. This ongoing research will aim to deepen the understanding of chromatin’s regulatory roles and its implications for various health conditions.
As scientists continue to explore the intricacies of the human genome, studies like this one shed light on the importance of considering the 3D architecture of DNA in understanding genetic regulation and disease mechanisms. The work of these researchers represents a significant step forward in the field of genomics, highlighting the need for a more nuanced approach to studying the human genome.






