Research published in Nature Communications has revealed a groundbreaking method for producing fertilizable human eggs using skin cells. This proof-of-concept study, led by Shoukhrat Mitapilov and colleagues at Oregon Health & Science University, suggests that cell reprogramming may provide a new avenue for addressing infertility, which currently impacts millions globally.
Infertility often arises from the dysfunction or absence of gametes, either oocytes (eggs) or sperm, which are essential for creating a zygote or fertilized egg. Traditional in vitro fertilization (IVF) methods may not be effective for all individuals, prompting scientists to explore alternative solutions such as somatic cell nuclear transfer (SCNT). This technique involves transplanting the nucleus from a skin cell into an enucleated donor egg, allowing the cell to develop into a functional oocyte.
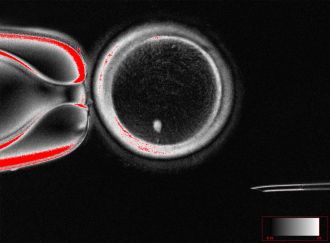
A significant challenge with this approach is that SCNT results in cells with a diploid genome, containing two sets of chromosomes, instead of the haploid set typical of standard gametes. To address this issue, the researchers developed a process named mitomeiosis, which mimics natural cell division by discarding one set of chromosomes. In their experiments, the team successfully produced 82 functional oocytes, which were fertilized in the laboratory. Approximately 9% of these fertilized eggs progressed to the blastocyst stage by day six post-fertilization, although no blastocysts survived beyond this point.
Despite the promising results, the researchers acknowledge limitations in their study. A majority of embryos did not advance past fertilization, and some exhibited chromosomal abnormalities. Nevertheless, this research signifies a potential leap forward in the field of reproductive medicine, showcasing the feasibility of generating eggs from somatic cells.
The process of SCNT allows for the direct reprogramming of somatic cells, creating functional oocytes. To facilitate ploidy reduction, the team investigated mitomeiosis, wherein somatic genomes undergo premature division when transplanted into enucleated oocytes. Initial fertilization attempts faced challenges, as SCNT oocytes remained arrested at the metaphase stage, indicating activation failure. However, artificial activation using a selective cyclin-dependent kinase inhibitor successfully triggered the segregation of chromosomes, leading to the development of embryos with integrated maternal and paternal genetic material.
The implications of this research are profound. As noted by Prof. Roger Sturmey, a Professor of Reproductive Medicine at the University of Hull, the ability to create new egg cells from differentiated adult cells could revolutionize treatment options for individuals facing infertility. He emphasized the importance of public dialogue regarding advancements in reproductive research and the need for robust governance to ensure accountability.
Prof. Ying Cheong from the University of Southampton remarked on the significance of this breakthrough, highlighting that for the first time, scientists have demonstrated that DNA from ordinary body cells can be manipulated to create functional gametes. This innovation could potentially transform the treatment landscape for individuals unable to use their own eggs due to age or medical conditions.
Additionally, Prof. Richard Anderson, Elsie Inglis Professor of Clinical Reproductive Science at the University of Edinburgh, noted the importance of this research for women who have lost their eggs, such as those affected by cancer treatments. The prospect of generating new eggs represents a potential major advance in reproductive health.
While this study marks a significant step forward, the authors caution that extensive research is still necessary to establish the efficacy and safety of these methods before they can be applied clinically. The potential for creating viable egg and sperm-like cells could open new doors for individuals facing infertility challenges, but further exploration is essential.
As the scientific community continues to investigate the capabilities of mitomeiosis and its applications, the possibility of revolutionizing reproductive medicine remains on the horizon.