Researchers at the Institute for Bioengineering of Catalonia (IBEC) have unveiled a revolutionary artificial cell that can navigate its environment using only chemical cues. This breakthrough, detailed in the journal Science Advances on July 25, 2025, marks a significant step in understanding how simple systems can exhibit complex behaviors similar to living organisms.
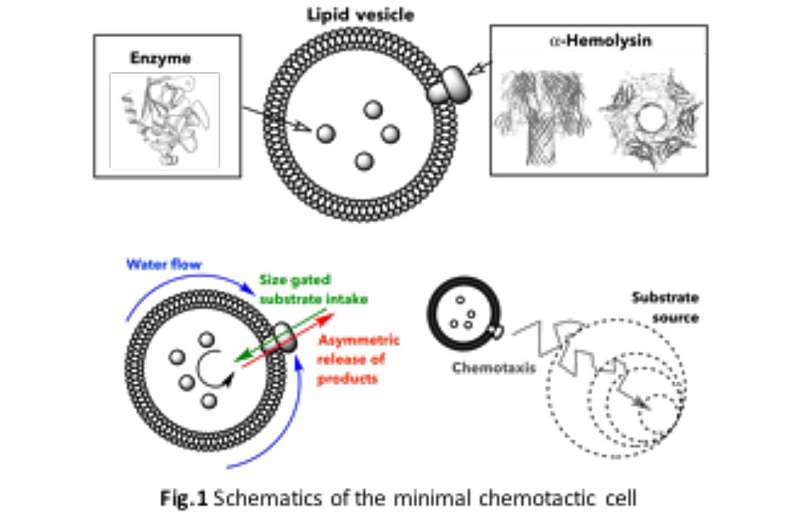
The newly created artificial cell, described as a “minimal cell,” consists of lipid vesicles encapsulating enzymes that enable it to propel itself through a process known as chemotaxis. This mechanism allows the artificial cell to move toward beneficial substances, mirroring the behavior observed in various living cells. Bárbara Borges Fernandes, a Ph.D. student at IBEC and the study’s lead author, notes that “bacteria rely on it to find food, white blood cells use it to reach sites of infection, and even sperm cells navigate toward the egg through chemotaxis.”
Understanding the Mechanism of Movement
The research team focused on how these cell-like vesicles operate in gradients of two key substrates: glucose and urea. By encapsulating glucose oxidase or urease enzymes within lipid-based vesicles, known as liposomes, they facilitated the conversion of glucose and urea into their respective end products. The team enhanced these liposomes by incorporating a crucial membrane pore protein, which serves as a channel for substrate entry and product exit.
The study highlights that active movement in these artificial cells relies on breaking symmetry. By trapping enzymes within the liposomes and utilizing the pores as exchange points, a difference in chemical concentration is generated around the particle. This creates fluid flow along the vesicle’s surface, effectively directing its movement. Borges explains, “It is as if the liposome were a boat, and the pore and the enzyme were its engine and navigation system.”
The researchers analyzed the behavior of over 10,000 vesicles within microfluidic channels under glucose or urease gradients. Their findings revealed that while control vesicles moved towards lower substrate concentrations due to passive effects, vesicles with increasing numbers of pores began to demonstrate active chemotaxis, ultimately reversing their direction towards areas of higher substrate concentration.
Implications for Synthetic Biology
The implications of this research extend beyond artificial cells. Understanding the principles of chemotaxis in a simplified system could provide insights into the evolution of more complex biological structures. Giuseppe Battaglia, a senior author and ICREA Research Professor at IBEC, remarked, “These synthetic cells are like blueprints for nature’s navigation system. Build simple, understand profoundly.”
The study was conducted in collaboration with the team of José Miguel Rubí at the University of Barcelona, who contributed theoretical predictions. The research also received support from the Institute for Physics of Living Systems, the Department of Chemistry at University College London, and the University of Liverpool, among others.
Battaglia emphasized the elegance of the research, stating, “We rebuild the whole dance with just three things: a fatty shell, one enzyme, and a pore. No fuss. Now the hidden rules jump out. That’s the power of synthetic biology: strip a puzzle down to its bones, and suddenly you see the music in the mess.”
This pioneering work not only lays the groundwork for future studies in synthetic biology but also opens new avenues for understanding cellular communication and function at a fundamental level. As scientists continue to explore these minimal systems, the potential for innovative applications, from drug delivery to environmental monitoring, remains vast.
For further information, refer to the article by Barbara Borges Fernandes et al, titled “The minimal chemotactic cell,” in Science Advances.






