A team of researchers from the Institute of Science Tokyo has developed an innovative tunable cell-sorting device that could revolutionize biomedical applications, particularly in cancer detection. By leveraging the unique properties of poly(N-isopropylacrylamide) (PNIPAM) hydrogel, the device offers the potential for high-resolution size-based sorting of cells from blood samples. Their findings were published in the journal Lab on a Chip on September 3, 2025.
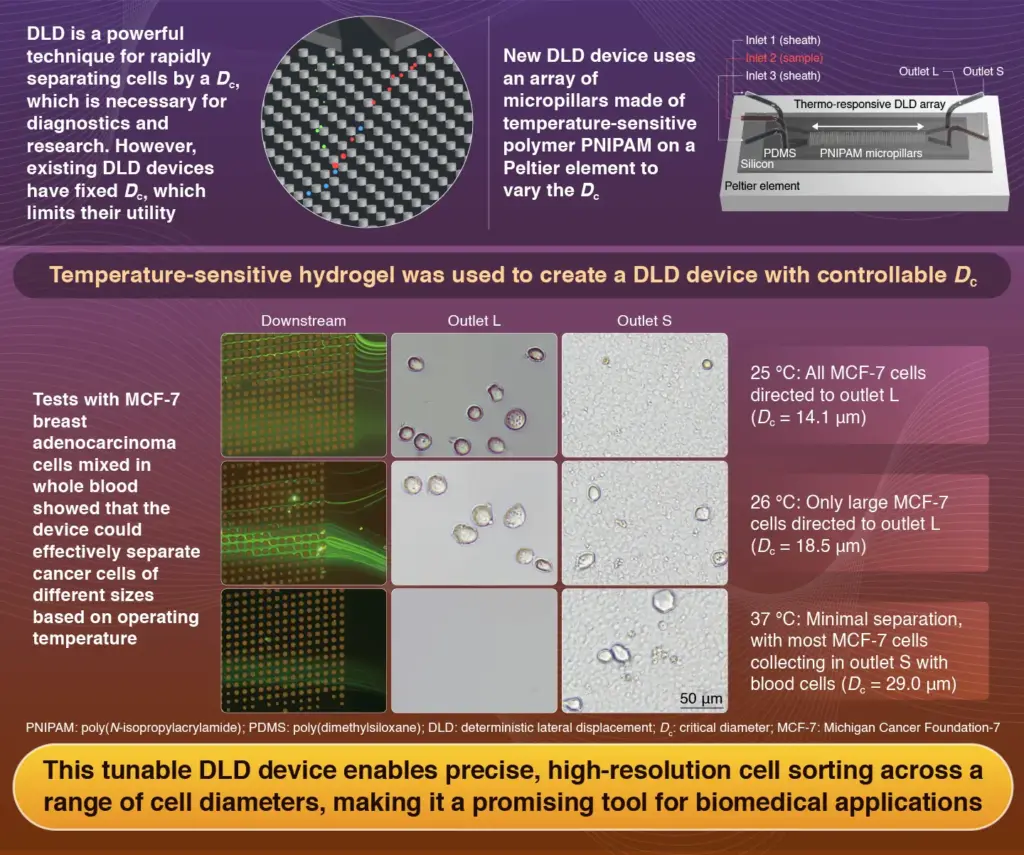
This tunable device utilizes a technology known as deterministic lateral displacement (DLD), which allows for the separation of cells based on size. Traditional DLD devices operate with a fixed critical diameter, limiting their application and efficiency. In contrast, the new platform adapts to temperature changes, altering its critical diameter between 20 °C and 40 °C to optimize sorting.
Advancements in Cell-Sorting Technology
The ability to isolate specific cell types from blood is vital for accurate medical diagnostics, especially in identifying metastatic cancer cells. DLD has gained popularity in recent years due to its high-throughput capabilities and the preservation of cell viability. The traditional DLD method, which uses micropillars to divert cells based on their size, faces challenges, including the risk of clogging from larger particles.
Led by Associate Professor Takasi Nisisako and Assistant Professor Yusuke Kanno, along with graduate student Ze Jiang, the research team developed a tunable DLD platform that overcomes these limitations. “In our previous work, we demonstrated a thermo-responsive DLD array on a glass substrate using hydrogel micropillars composed of PNIPAM within a poly(dimethylsiloxane) (PDMS) microchannel,” Nisisako explains. He emphasizes the simplicity of their approach, which eliminates the need for complex external equipment and allows for direct temperature-driven modulation of pillar dimensions.
The latest iteration of the DLD device features a silicon base mounted on a Peltier element, enhancing thermal control. Liquid samples are directed through plasma-bonded PDMS microchannels to the PNIPAM microarray. The device is equipped with two outlets—designated L and S—at the end of the array, facilitating the separation of sorted cells.
Successful Testing and Future Applications
The research team validated the tunability of their device by using blood samples mixed with Michigan Cancer Foundation-7 (MCF-7) breast adenocarcinoma cells, which average 17 μm in diameter—significantly larger than typical blood cells. When the sample was processed at 25 °C (with a critical diameter of 14.1 μm), the device achieved a sorting efficiency of 90% for MCF-7 cells into outlet L. As the temperature increased to 26 °C and 37 °C, the distribution of cells shifted, demonstrating the device’s adaptability to different critical diameters.
Nisisako expresses enthusiasm about the potential of this technology. “The precision, versatility, and reliability of this platform underscore its potential for high-resolution size-based sorting, making it a promising tool for a wide range of biomedical applications.” The team now aims to further evaluate the device’s effectiveness with actual patient samples, potentially paving the way for enhanced cancer diagnostics and treatment strategies.
This breakthrough in cell-sorting technology marks a significant advancement in the field of biomedical research, with implications that could extend far beyond cancer detection.